Acid base titration curve pdf unveils the secrets of acid-base reactions, offering a visual roadmap through the fascinating world of chemistry. This comprehensive guide explores the fundamental principles, practical techniques, and real-world applications of acid-base titrations. From understanding the crucial role of indicators to mastering the art of accurate measurements, the curve reveals a wealth of information about the solutions it examines.
Get ready to embark on a journey of discovery, unraveling the mysteries hidden within these intricate chemical processes.
This guide walks you through the process of constructing, interpreting, and applying titration curves. It details the meticulous steps involved, including calculating pH values, preparing standard solutions, and identifying equivalence points. The document further delves into troubleshooting common errors and offers valuable insights into the diverse applications of acid-base titrations across various scientific disciplines.
Introduction to Acid-Base Titration Curves: Acid Base Titration Curve Pdf
Acid-base titrations are fundamental experiments in chemistry, allowing us to precisely determine the concentration of an unknown acid or base. Imagine trying to figure out the exact strength of a cleaning solution—a titration helps us quantify that strength. This process is crucial for understanding chemical reactions and their quantitative aspects. It’s like finding the perfect balance in a recipe, ensuring the right amount of each ingredient.The fundamental principle behind acid-base reactions lies in the transfer of protons (H+ ions).
Strong acids readily donate protons, while strong bases readily accept them. The reaction is essentially a neutralization process, where the acid and base react to form water and a salt. This process is highly predictable and quantifiable, making it useful for various applications, from laboratory analysis to industrial processes. Titration curves visually represent the change in pH as a base is added to an acid, or vice versa.
Fundamental Principles of Acid-Base Reactions
Acid-base reactions involve the transfer of protons (H+ ions). Strong acids completely dissociate in water, releasing all their protons. Weak acids only partially dissociate. Similarly, strong bases completely ionize in water, releasing hydroxide ions (OH-), while weak bases only partially ionize. The strength of an acid or base dictates how readily it donates or accepts protons, respectively.
Understanding these principles is vital to interpreting titration curves.
Components of a Typical Acid-Base Titration Setup
A typical acid-base titration setup involves several key components. These components work together to allow for accurate measurement and analysis of the reaction. This precision is crucial for determining the concentration of the unknown solution.
| Component | Description | Function |
|---|---|---|
| Burette | A graduated glass tube with a stopcock. | Used to precisely deliver a known volume of titrant (the solution of known concentration). |
| Erlenmeyer Flask | A conical flask used to hold the analyte (the solution with unknown concentration). | Contains the solution being analyzed. |
| Indicator | A substance that changes color at a specific pH range. | Signals the endpoint of the titration by changing color. |
| Stand | A sturdy support for the burette | Maintains the stability of the burette. |
Role of Indicators in Acid-Base Titrations
Indicators are substances that exhibit distinct color changes at specific pH values. These color changes mark the endpoint of the titration, which is the point where the reaction is complete. Choosing the appropriate indicator is crucial, as the color change must occur close to the equivalence point (the point where the moles of acid and base are stoichiometrically equal).
Different indicators have different color changes at different pH ranges, allowing for different types of titrations. For example, phenolphthalein changes color in a slightly basic range, while methyl orange changes color in a more acidic range. This allows chemists to select an indicator that accurately reflects the reaction’s endpoint.
Constructing Acid-Base Titration Curves
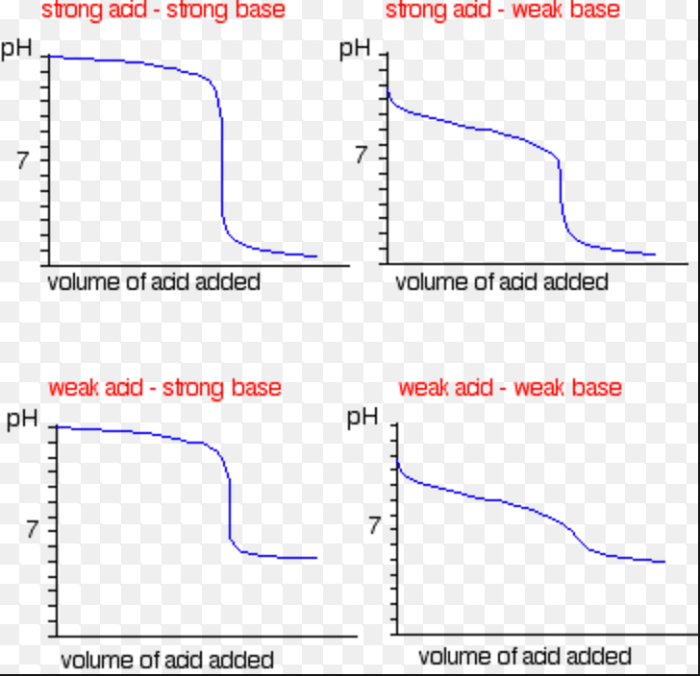
Unveiling the secrets of acid-base reactions often involves meticulously charting their progress. A titration curve, a graphical representation of pH changes during a titration, provides invaluable insights into the nature of the reaction. It’s a roadmap to understanding the strength of acids and bases and the equilibrium dynamics at play.Plotting a titration curve involves a series of careful steps, from precisely measuring volumes to calculating pH values.
Accurate measurements are paramount, as small errors can lead to significant deviations in the curve’s shape. Understanding these steps and the calculations behind them empowers us to interpret the data effectively and draw meaningful conclusions.
Steps Involved in Plotting a Titration Curve
This section details the crucial steps involved in creating a titration curve, a visual representation of the acid-base reaction’s progression. Careful attention to detail is essential for reliable results.
- Prepare a solution of known concentration (the titrant) and measure a precise volume of the unknown solution (the analyte). The titrant is added incrementally to the analyte.
- Measure the pH of the solution after each addition of titrant. Use a pH meter or indicator solution for accurate pH determination.
- Plot the pH values against the volume of titrant added. This generates the titration curve.
Calculating pH Values
Accurate pH calculations are essential for accurately interpreting the titration curve. The method employed depends on the stage of the titration.
- Before the equivalence point, the pH is primarily determined by the concentration of the analyte. Calculations involving the initial concentration and the volume of titrant added are crucial.
- At the equivalence point, the moles of acid and base are equal. The pH at this point depends on the nature of the acid and base. Calculations often involve the use of the Ka or Kb values.
- Beyond the equivalence point, the pH is primarily determined by the excess titrant. Calculations using the concentration and volume of the excess titrant are necessary.
Importance of Accurate Measurements
Precise measurements are vital for accurate results in any titration experiment. This precision ensures the reliability of the titration curve and the conclusions drawn from it.
- Precise volume measurements are crucial. Use calibrated glassware like burets and pipettes to minimize errors.
- Accurate concentration of the titrant is essential. Standardization procedures are critical to ensure accuracy.
- Regular calibration of the pH meter ensures accurate pH readings.
Preparing a Standardized Solution
A standardized solution is one whose concentration is precisely known. This is crucial for accurate titrations.
- Weighing: Accurately weigh a precise amount of primary standard (a substance with high purity and known stoichiometry) using an analytical balance.
- Dissolving: Dissolve the weighed primary standard in a known volume of solvent, usually distilled water.
- Calculating: Calculate the concentration of the resulting solution using the formula: Concentration = (mass of solute / molar mass of solute) / volume of solution.
Contrasting pH Changes During Titrations
The following table summarizes the pH changes during the titration of various acid-base combinations.
| Acid/Base | pH Change | Equivalence Point |
|---|---|---|
| Strong Acid with Strong Base | Sharp increase around equivalence point | Around pH 7 |
| Weak Acid with Strong Base | Gradual increase around equivalence point | Above pH 7 |
| Weak Base with Strong Acid | Gradual decrease around equivalence point | Below pH 7 |
Interpreting Acid-Base Titration Curves
Unveiling the secrets hidden within the elegant curves of acid-base titrations is like deciphering a hidden code. These curves, meticulously plotted, reveal critical information about the reaction’s progress and the nature of the interacting substances. They are more than just pretty graphs; they’re powerful tools for understanding acid-base chemistry.The equivalence point, a pivotal moment in the titration, signifies the precise stoichiometric balance between the acid and base.
It’s like the ultimate handshake, where the reactants have perfectly neutralized each other. The characteristics of the titration curves for strong and weak acids/bases vary dramatically, mirroring the inherent differences in their chemical personalities. Knowing how to identify this crucial equivalence point on a titration curve is paramount. The curves themselves act as silent storytellers, offering valuable insights into the strength and nature of the reacting substances.
Comparing and contrasting these curves is like comparing the contrasting personalities of two friends, revealing the underlying chemistry that drives their reactions.
Significance of Equivalence Points, Acid base titration curve pdf
The equivalence point marks the stoichiometric point of the reaction. This is where the moles of acid exactly equal the moles of base, resulting in a neutral solution (pH = 7) for a strong acid/strong base reaction. For reactions involving a weak acid or base, the pH at the equivalence point will deviate from 7. Understanding this crucial point is essential for accurate analysis and determining the concentration of unknown solutions.
Knowing the exact volume of titrant needed to reach the equivalence point allows for precise calculations.
Characteristics of Titration Curves for Strong and Weak Acids/Bases
Strong acids and bases completely dissociate in water, leading to steep, vertical changes in pH near the equivalence point. This abrupt shift is easily observed on the titration curve. Weak acids and bases, on the other hand, only partially dissociate, resulting in a gentler, more gradual change in pH near the equivalence point. The shape of the curve reflects the equilibrium constant for the dissociation of the weak acid or base.
Identifying the Equivalence Point on a Titration Curve
The equivalence point is the point on the titration curve where the steepest change in pH occurs. It’s the inflection point. Visualizing the curve helps. A sharp, sudden rise or fall in pH near the equivalence point clearly indicates the reaction’s completion. This point on the graph allows for the precise determination of the concentration of the unknown solution.
Comparing and Contrasting Titration Curves of Strong and Weak Acids
Strong acids exhibit sharp, vertical changes in pH near the equivalence point, reflecting their complete dissociation. Weak acids, in contrast, show a more gradual shift in pH, indicating incomplete dissociation. This difference in the curve’s shape directly corresponds to the inherent strength of the acid. The curves offer a visual representation of the chemical nature of the reactants.
Summary of Key Features of Titration Curves
| Type of Acid/Base | Shape of Curve Near Equivalence Point | pH at Equivalence Point | Buffer Region |
|---|---|---|---|
| Strong Acid/Strong Base | Sharp, vertical change | 7 | Very small |
| Strong Acid/Weak Base | Sharp, vertical change | Less than 7 | Small |
| Weak Acid/Strong Base | Gradual change | Greater than 7 | Significant |
| Weak Acid/Weak Base | Gradual change | Variable (Depends on the acid/base) | Significant |
Applications of Acid-Base Titration Curves
Acid-base titrations, a cornerstone of analytical chemistry, aren’t just theoretical exercises. They’re powerful tools with real-world applications, from determining the acidity of soil to ensuring the quality of pharmaceuticals. Understanding how titration curves work unlocks the secrets hidden within these chemical reactions.Titration curves provide a visual roadmap of the reaction between an acid and a base. This roadmap reveals critical information about the unknown solution, such as its concentration and identity.
The shape of the curve, particularly the steep, vertical portion, offers clues to the nature of the reactants involved.
Determining the Concentration of Unknown Solutions
Knowing the concentration of a solution is crucial in many fields. Titration curves offer a precise and reliable method to determine unknown concentrations. By carefully measuring the volume of a standard solution required to neutralize the unknown, the concentration of the unknown solution can be calculated. This process is essential in industries like food processing and environmental monitoring, where accurate measurements of acidity or alkalinity are critical.
Identifying Unknown Acids or Bases
The shape of a titration curve is highly dependent on the nature of the acid or base being titrated. Strong acids and bases yield distinctive curves compared to weak ones. The equivalence point, a pivotal point on the curve, can help identify the unknown acid or base. The characteristics of the curve, including the steepness and location of the equivalence point, provide valuable information to determine the identity of the unknown substance.
Real-World Applications of Acid-Base Titrations
Acid-base titrations find extensive applications in various fields, demonstrating their broad utility. They’re indispensable in quality control in pharmaceutical industries, ensuring that medications have the correct strength and purity. Environmental scientists use titrations to monitor the acidity of lakes and streams, ensuring the health of aquatic ecosystems. In food science, titrations determine the acidity of fruits and vegetables, which influences their taste and preservation.
Scenarios Where Titration Curves Are Essential
Titration curves are indispensable in numerous scenarios. For example, in a pharmaceutical lab, verifying the purity and concentration of a medication is paramount, where titration curves are vital. Similarly, determining the level of acidity in industrial wastewater is crucial to prevent environmental damage, making titration curves essential in environmental monitoring. Moreover, in a chemistry lab, identifying the type of acid or base in an unknown solution relies on the unique features of the titration curve.
Table of Applications in Different Fields
| Field | Application |
|---|---|
| Chemistry | Determining the concentration of unknown acids and bases, identifying unknown substances, and studying acid-base reactions. |
| Environmental Science | Monitoring water quality (acidity of lakes and streams), analyzing soil samples, and studying pollution levels. |
| Food Science | Determining the acidity of fruits and vegetables, analyzing food products for proper preservation and taste. |
| Medicine | Quality control of medications, ensuring correct dosage and purity. |
Common Errors and Troubleshooting
Navigating the intricacies of acid-base titrations can sometimes feel like a delicate dance. Understanding potential pitfalls and how to address them is key to achieving accurate results. This section will illuminate common mistakes, troubleshooting strategies, and factors affecting precision, empowering you to become a titration maestro.
Identifying and Mitigating Errors
Errors in acid-base titrations can stem from various sources, ranging from imprecise measurements to flawed techniques. Carefully considering these factors is crucial for reliable results. Inaccurate initial readings, faulty equipment, or even environmental fluctuations can all influence the outcome. Addressing these issues systematically will significantly improve the accuracy and reliability of your titration experiments.
Imprecision in Measurement
Accurate measurements are the cornerstone of precise titrations. Errors in measuring reagents, particularly the titrant, can significantly skew the results. Using calibrated glassware, ensuring proper technique, and minimizing parallax errors are essential. A poorly calibrated buret, for example, can lead to inaccurate volume readings, resulting in an incorrect equivalence point determination.
Troubleshooting Common Problems
Sometimes, despite meticulous preparation, unexpected issues can arise during a titration. Understanding the causes and remedies for these problems is vital. For instance, if the solution’s color change is sluggish or erratic, a slow addition of titrant or a different indicator might be the solution.
Factors Affecting Accuracy
Several factors can influence the accuracy of titration results. These include the quality of reagents, the suitability of the indicator, and the presence of interfering substances. Using high-purity reagents and selecting an appropriate indicator that changes color at the equivalence point are crucial for minimizing errors. Contaminants in the sample or the presence of impurities in the reagents can lead to inaccuracies in the calculations.
Minimizing Errors in the Titration Process
Minimizing errors in the titration process requires a meticulous approach. Careful technique, consistent procedures, and thorough documentation are crucial. For instance, controlling the rate of titrant addition is vital to ensure a sharp endpoint. This careful attention to detail is what separates a good titration from a great one.
Tips for Precise Measurement and Accurate Data Collection
To ensure the accuracy of your titration results, meticulous attention to detail is essential.
- Use calibrated glassware for precise volume measurements.
- Employ a standardized technique for adding titrant.
- Observe the color change carefully and accurately.
- Record all observations and measurements meticulously.
- Repeat the titration process multiple times to verify the results and calculate the average value.
- Control environmental factors, such as temperature and humidity, to minimize their impact.
- Thoroughly clean glassware to prevent contamination and ensure accurate results.
Visual Representations of Titration Curves

Acid-base titrations are like a chemist’s dance, where you carefully add one solution to another to precisely measure the amount of one substance in another. The titration curve is the choreographer’s notes, revealing the dance’s steps and the fascinating chemistry happening during the reaction. These curves are powerful tools for understanding acid-base reactions and determining unknown concentrations.Visualizing these reactions through titration curves provides a clearer picture of the acid-base chemistry taking place.
Each curve, a unique story of proton transfer, tells us a lot about the nature of the acids and bases involved. We can analyze the shape and features of these curves to determine the equivalence point, a crucial landmark in the reaction.
Strong Acid/Strong Base Titration
Strong acids and strong bases completely ionize in water. The titration curve of a strong acid titrated with a strong base exhibits a sharp, vertical rise in pH near the equivalence point. This sharp change indicates a rapid neutralization of the acid by the base. The equivalence point is usually near pH 7, indicating a neutral solution.
Weak Acid/Strong Base Titration
Weak acids, unlike their strong counterparts, don’t fully ionize. The titration curve of a weak acid titrated with a strong base shows a less dramatic but still significant change in pH. The curve has a gentle upward slope at the beginning, followed by a more substantial rise in pH around the equivalence point. This gentle slope before the equivalence point arises because the weak acid’s conjugate base is a weak base, so the solution resists the pH change.
The equivalence point, typically above pH 7, reflects the basic nature of the resulting solution.
Weak Base/Strong Acid Titration
The titration curve of a weak base with a strong acid is the mirror image of the weak acid/strong base titration. The curve initially has a gentle downward slope before the equivalence point, where the solution resists the pH change. The sharp change in pH around the equivalence point occurs, but the equivalence point is below pH 7, reflecting the acidic nature of the resulting solution.
Strong Base/Strong Acid Titration: A Closer Look
Let’s examine the curve for a strong base titrated with a strong acid. The initial pH of the strong base solution is high, and as the strong acid is added, the pH gradually decreases. The graph shows a steep vertical drop in pH near the equivalence point. This rapid change indicates the rapid neutralization of the base by the acid.
The equivalence point typically occurs around pH 7.
pH vs. Volume of Titrant Added
The relationship between pH and the volume of titrant added is fundamental to understanding titration curves. The shape of the curve reveals how the pH changes as the titrant is added. The steepest part of the curve near the equivalence point corresponds to the most rapid change in pH. The curve’s shape is intimately connected to the nature of the acid and base involved.
This relationship is crucial in determining the equivalence point and the concentration of the unknown solution.
Comprehensive Acid-Base Titration Curve
| Volume of Titrant (mL) | pH |
|---|---|
| 0 | 13.0 |
| 10 | 12.5 |
| 20 | 11.0 |
| 30 | 10.0 |
| 40 | 9.5 |
| 50 | 9.0 |
| 60 | 8.0 |
| 70 | 7.0 |
| 80 | 6.5 |
| 90 | 6.0 |
| 100 | 5.5 |
This table provides a hypothetical example of a strong base titrated with a strong acid. The curve shows a rapid change in pH around the equivalence point. The table shows how the pH changes as the volume of titrant is added, visually representing the titration process.
