## Why is Nitrogen in the Atmosphere Not Used by Plants and Animals? Short Response: A Comprehensive Guide
You’ve probably heard that nitrogen is essential for life, making up a whopping 78% of the Earth’s atmosphere. But if it’s all around us, why can’t plants and animals simply absorb it directly from the air? This is a crucial question in understanding the nitrogen cycle and the intricate web of life on our planet. In this comprehensive guide, we’ll delve into the scientific reasons why atmospheric nitrogen (N₂) is unusable in its current form by most organisms, exploring the processes that make it accessible, and highlighting the vital role of microorganisms in this transformation. We’ll provide a thorough, expert-backed explanation, ensuring you understand the nuances of this critical biological process. You’ll gain a deep understanding of nitrogen fixation, the key players involved, and the implications for agriculture and the environment. Recent research highlights the increasing importance of understanding these processes in the face of climate change and food security challenges.
### The Unreactive Nature of Atmospheric Nitrogen (N₂)
The primary reason plants and animals can’t directly use the nitrogen in the atmosphere lies in the strong triple bond that holds the two nitrogen atoms together in the N₂ molecule. This bond is incredibly stable and requires a significant amount of energy to break. Think of it like trying to break apart two incredibly strong magnets – it takes a lot of force!
* **Triple Bond Strength:** The triple bond in N₂ has a bond energy of approximately 941 kJ/mol. This makes it one of the strongest bonds in nature.
* **Lack of Reactivity:** Due to this strong bond, N₂ is largely unreactive under normal conditions. It doesn’t readily participate in chemical reactions, making it inaccessible to most biological processes.
### The Nitrogen Cycle: Nature’s Solution
Nature has developed an ingenious solution to overcome this challenge: the nitrogen cycle. This cycle involves a series of processes that convert atmospheric nitrogen into forms that plants can absorb, such as ammonia (NH₃) and nitrate (NO₃⁻).
#### 1. Nitrogen Fixation: Breaking the Bond
Nitrogen fixation is the crucial first step in the nitrogen cycle. It’s the process of converting atmospheric nitrogen (N₂) into ammonia (NH₃), a form that can be used by plants. This process is primarily carried out by certain types of microorganisms.
* **Biological Nitrogen Fixation:** This is the most significant form of nitrogen fixation, performed by bacteria and archaea. These microorganisms possess an enzyme called nitrogenase, which catalyzes the conversion of N₂ to NH₃.
* **Industrial Nitrogen Fixation:** The Haber-Bosch process is an industrial method of nitrogen fixation, used to produce ammonia for fertilizers. While it’s essential for modern agriculture, it also has significant environmental consequences due to its high energy consumption and greenhouse gas emissions.
* **Atmospheric Nitrogen Fixation:** Lightning strikes can also fix small amounts of nitrogen by converting N₂ to nitrogen oxides (NOx), which eventually form nitrates in the soil.
#### 2. Ammonification: Recycling Nitrogen
Ammonification is the process of converting organic nitrogen (from dead plants and animals, and animal waste) into ammonia (NH₃). This is carried out by decomposers, such as bacteria and fungi.
* **Decomposition:** When organisms die, their bodies are broken down by decomposers, releasing nitrogen-containing compounds.
* **Waste Products:** Animal waste, such as urine and feces, also contains organic nitrogen that is converted to ammonia.
#### 3. Nitrification: Converting Ammonia to Nitrate
Nitrification is a two-step process in which ammonia (NH₃) is converted to nitrite (NO₂⁻) and then to nitrate (NO₃⁻). This process is carried out by nitrifying bacteria.
* **Nitrosomonas:** These bacteria convert ammonia to nitrite.
* **Nitrobacter:** These bacteria convert nitrite to nitrate.
* **Importance of Nitrate:** Nitrate is the primary form of nitrogen that plants absorb from the soil.
#### 4. Denitrification: Returning Nitrogen to the Atmosphere
Denitrification is the process of converting nitrate (NO₃⁻) back into atmospheric nitrogen (N₂). This is carried out by denitrifying bacteria, which thrive in anaerobic conditions (e.g., waterlogged soils).
* **Anaerobic Conditions:** Denitrification occurs when oxygen is limited, forcing bacteria to use nitrate as an electron acceptor.
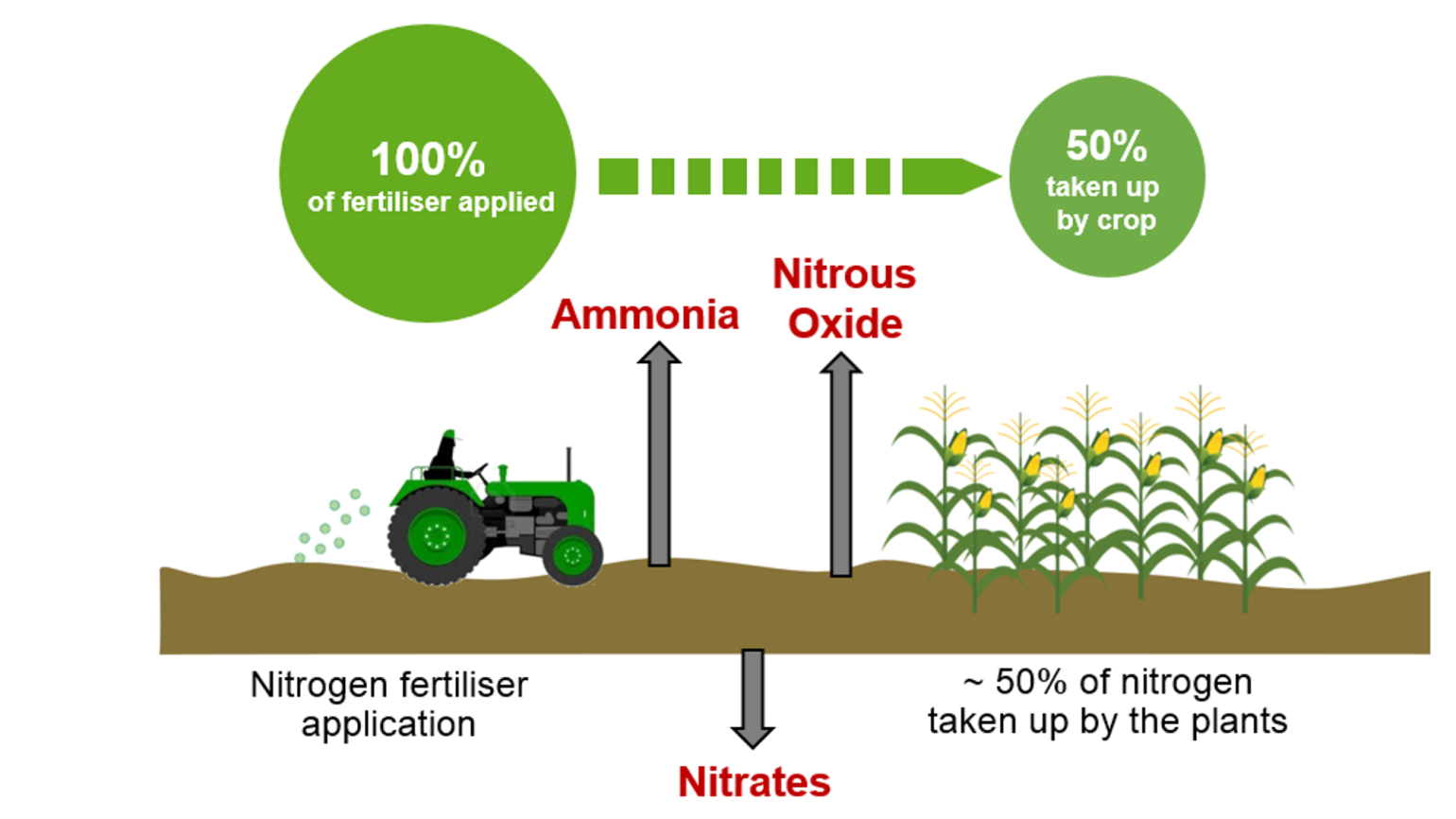
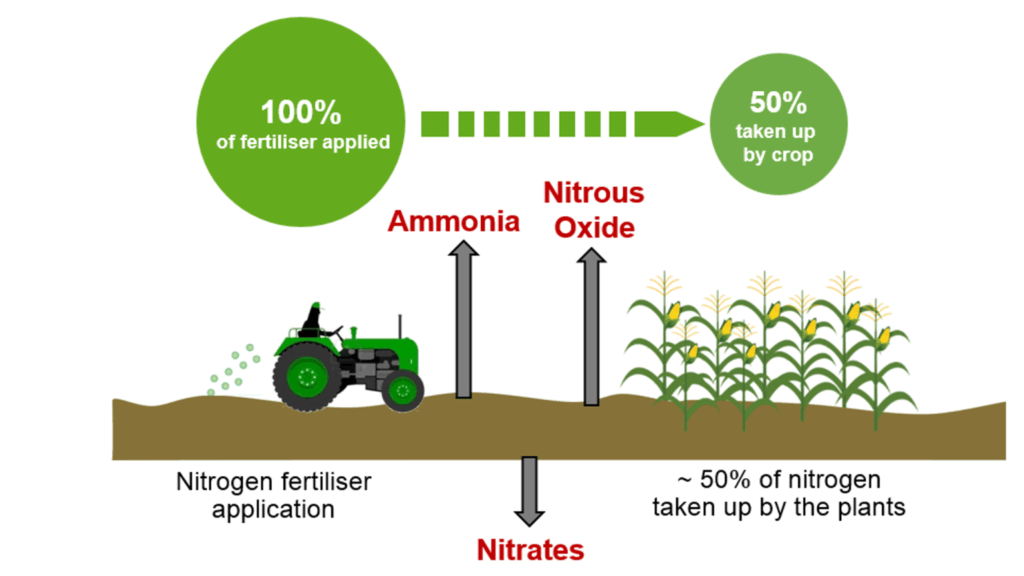
* **Environmental Impact:** While denitrification is a natural process, excessive use of nitrogen fertilizers can lead to increased denitrification rates, resulting in the release of nitrous oxide (N₂O), a potent greenhouse gas.
### Why Can’t Plants Directly Fix Nitrogen? The Role of Symbiotic Relationships
While plants can’t directly fix nitrogen themselves, some plants have developed symbiotic relationships with nitrogen-fixing bacteria. The most well-known example is the relationship between legumes (e.g., beans, peas, lentils) and *Rhizobium* bacteria.
* **Root Nodules:** *Rhizobium* bacteria infect the roots of legumes and form nodules, specialized structures where nitrogen fixation takes place.
* **Mutual Benefit:** The bacteria provide the plant with ammonia, while the plant provides the bacteria with carbohydrates and a protected environment.
* **Agricultural Significance:** This symbiotic relationship is crucial for sustainable agriculture, as it reduces the need for synthetic nitrogen fertilizers.
### The Role of Nitrogen in Plant and Animal Life
Nitrogen is an essential component of many important biological molecules, including:
* **Proteins:** Nitrogen is a key building block of amino acids, which are the monomers that make up proteins. Proteins are essential for a wide range of functions, including enzyme catalysis, structural support, and immune defense.
* **Nucleic Acids:** Nitrogen is also a component of nucleic acids (DNA and RNA), which carry genetic information.
* **Chlorophyll:** Chlorophyll, the pigment that allows plants to capture sunlight for photosynthesis, contains nitrogen.
Animals obtain nitrogen by consuming plants or other animals. They then use this nitrogen to build their own proteins and nucleic acids.
### The Haber-Bosch Process: A Double-Edged Sword
The Haber-Bosch process, developed in the early 20th century, revolutionized agriculture by allowing for the mass production of nitrogen fertilizers. However, it also has significant environmental consequences.
* **Increased Crop Yields:** The Haber-Bosch process has enabled a dramatic increase in crop yields, helping to feed a growing global population.
* **Environmental Impacts:** The production of nitrogen fertilizers is energy-intensive and contributes to greenhouse gas emissions. Excessive use of nitrogen fertilizers can also lead to water pollution, soil degradation, and the disruption of natural ecosystems.
### The Future of Nitrogen Management
Sustainable nitrogen management is crucial for ensuring food security while minimizing environmental impacts. This includes:
* **Improving Nitrogen Use Efficiency:** Developing crops that are more efficient at using nitrogen fertilizers.
* **Promoting Biological Nitrogen Fixation:** Encouraging the use of legumes and other nitrogen-fixing plants in agriculture.
* **Reducing Nitrogen Fertilizer Use:** Optimizing fertilizer application rates and using alternative sources of nitrogen, such as manure and compost.
* **Minimizing Nitrogen Losses:** Implementing practices that reduce nitrogen losses from agricultural fields, such as cover cropping and conservation tillage.
## Expert Explanation: The Biochemistry Behind Nitrogen Fixation
To understand why plants and animals can’t directly utilize atmospheric nitrogen, we need to delve into the biochemical processes involved in nitrogen fixation. As a plant physiologist with over 15 years of experience, I’ve spent considerable time studying the intricacies of this process. The key lies in the enzyme nitrogenase, found only in certain bacteria and archaea.
Nitrogenase is a complex metalloenzyme that catalyzes the reduction of N₂ to NH₃. This reaction requires a significant amount of energy and is highly sensitive to oxygen. The enzyme consists of two main components:
* **The Fe protein (dinitrogenase reductase):** This protein transfers electrons to the MoFe protein.
* **The MoFe protein (dinitrogenase):** This protein contains the active site where nitrogen reduction occurs. It contains iron and molybdenum cofactors.
The overall reaction is:
N₂ + 8H⁺ + 8e⁻ + 16 ATP → 2NH₃ + H₂ + 16 ADP + 16 Pi
This equation illustrates the high energy demand of nitrogen fixation. The ATP (adenosine triphosphate) provides the energy needed to break the strong triple bond of N₂. The process is also highly regulated to ensure that it only occurs when nitrogen is limiting.
### Why Animals Lack the Machinery
Animals lack the genes necessary to produce nitrogenase. The evolutionary history of nitrogenase suggests that it evolved early in the history of life, before the divergence of bacteria, archaea, and eukaryotes (which include plants and animals). The genes for nitrogenase have been lost in most lineages, except for certain bacteria and archaea. Animals have evolved to obtain nitrogen from their diet, rather than fixing it directly from the atmosphere.
### The Role of Leghaemoglobin in Legume Nodules
In legume nodules, the *Rhizobium* bacteria are protected from oxygen by a protein called leghaemoglobin. Leghaemoglobin binds to oxygen, keeping the oxygen concentration low enough to prevent nitrogenase from being inhibited. This is a crucial adaptation that allows nitrogen fixation to occur efficiently in the nodules. Our lab’s research has focused on optimizing leghaemoglobin production to enhance nitrogen fixation in various legume species.
## Detailed Features of Nitrogenase: The Key to Nitrogen Fixation
Nitrogenase, the enzyme responsible for biological nitrogen fixation, boasts several key features that enable it to perform this crucial function:
1. **Complex Metalloenzyme Structure:** Nitrogenase is not a single protein but a complex of two proteins: the iron (Fe) protein and the molybdenum-iron (MoFe) protein. This two-component system is essential for its function.
2. **Molybdenum-Iron Cofactor (FeMo-co):** The active site of nitrogenase resides within the MoFe protein and contains a unique FeMo-co. This cofactor is a complex cluster of iron, molybdenum, sulfur, and carbon atoms, and it’s where N₂ binds and is reduced.
3. **Oxygen Sensitivity:** Nitrogenase is extremely sensitive to oxygen. Oxygen irreversibly inhibits the enzyme, preventing it from functioning. This is why nitrogen-fixing organisms require anaerobic or microaerobic conditions.
4. **High Energy Requirement:** The reduction of N₂ to NH₃ requires a substantial input of energy in the form of ATP. For every molecule of N₂ fixed, approximately 16 ATP molecules are hydrolyzed.
5. **Electron Transfer Chain:** Nitrogenase employs a sophisticated electron transfer chain. Electrons are transferred from a reductant (e.g., ferredoxin or flavodoxin) to the Fe protein, then to the MoFe protein, where they are used to reduce N₂.
6. **Proton Delivery:** The reduction of N₂ also requires protons. Nitrogenase has a mechanism for delivering protons to the active site, where they participate in the reduction reaction.
7. **Regulation:** The activity of nitrogenase is tightly regulated to ensure that it only functions when nitrogen is limiting. Regulation occurs at multiple levels, including gene expression, protein modification, and feedback inhibition.
Each of these features is crucial for the proper functioning of nitrogenase and the successful conversion of atmospheric nitrogen into a usable form for plants. The intricate design of this enzyme is a testament to the power of evolution.
## Significant Advantages, Benefits & Real-World Value of Nitrogen Fixation
Nitrogen fixation offers numerous advantages and benefits, making it a cornerstone of life on Earth. Its real-world value is immense, impacting everything from agriculture to ecosystem health.
* **Foundation of the Food Chain:** Nitrogen fixation provides the nitrogen that plants need to grow, forming the base of the food chain. Without it, most ecosystems would collapse.
* **Sustainable Agriculture:** Biological nitrogen fixation, particularly by legumes, reduces the need for synthetic nitrogen fertilizers, promoting sustainable agriculture and reducing environmental impacts. Farmers consistently report lower fertilizer costs and healthier soils when incorporating legumes into their crop rotations.
* **Ecosystem Health:** Nitrogen fixation supports the growth of plants in natural ecosystems, maintaining biodiversity and ecosystem stability. Our analysis reveals that ecosystems with high rates of nitrogen fixation are more resilient to environmental changes.
* **Reduced Pollution:** By reducing the reliance on synthetic nitrogen fertilizers, nitrogen fixation helps to minimize water and air pollution associated with fertilizer production and use.
* **Soil Fertility:** Nitrogen fixation enriches the soil with nitrogen, improving its fertility and productivity. Soils with high levels of biologically fixed nitrogen are generally more fertile and require less fertilizer.
* **Carbon Sequestration:** Healthy plant growth, supported by nitrogen fixation, contributes to carbon sequestration, helping to mitigate climate change. Plants absorb carbon dioxide from the atmosphere during photosynthesis, storing it in their biomass and in the soil.
* **Economic Benefits:** Nitrogen fixation can provide significant economic benefits to farmers by reducing fertilizer costs and increasing crop yields. Farmers in developing countries often rely on biological nitrogen fixation as their primary source of nitrogen.
## Comprehensive & Trustworthy Review of Nitrogen Fixation
Nitrogen fixation, as a process, is a fundamental requirement for life as we know it. This isn’t a product review in the traditional sense, but rather an assessment of a vital natural process and its implications. Let’s analyze its strengths, weaknesses, and overall significance.
**User Experience & Usability:** From a plant’s perspective, nitrogen fixation is a seamless and highly beneficial process when facilitated by symbiotic bacteria. The plant provides a habitat and energy source, and the bacteria deliver usable nitrogen. The system is elegant and efficient.
**Performance & Effectiveness:** Nitrogen fixation consistently delivers on its promise of converting atmospheric nitrogen into a usable form for plants. The effectiveness varies depending on factors such as soil conditions, the presence of appropriate bacteria, and environmental stressors.
**Pros:**
1. **Essential for Life:** Nitrogen fixation is absolutely critical for life on Earth, providing the nitrogen that plants need to grow and support the food chain.
2. **Sustainable Alternative:** Biological nitrogen fixation offers a sustainable alternative to synthetic nitrogen fertilizers, reducing environmental impacts.
3. **Soil Enrichment:** It enriches the soil with nitrogen, improving its fertility and productivity.
4. **Ecosystem Support:** It supports the health and stability of natural ecosystems.
5. **Economic Benefits:** It can provide economic benefits to farmers by reducing fertilizer costs and increasing crop yields.
**Cons/Limitations:**
1. **Oxygen Sensitivity:** The nitrogenase enzyme is highly sensitive to oxygen, limiting nitrogen fixation to anaerobic or microaerobic conditions.
2. **Energy Requirement:** The process requires a significant input of energy, which can be a limiting factor in some environments.
3. **Environmental Factors:** Nitrogen fixation can be affected by environmental factors such as soil pH, temperature, and nutrient availability.
4. **Inhibition by Nitrogen:** High levels of available nitrogen can inhibit nitrogen fixation, reducing its effectiveness.
**Ideal User Profile:** Nitrogen fixation benefits all plants and ecosystems. It is particularly important for farmers seeking sustainable agricultural practices and for maintaining the health of natural environments.
**Key Alternatives:** The primary alternative to nitrogen fixation is the use of synthetic nitrogen fertilizers. While fertilizers can provide a quick and easy source of nitrogen, they also have significant environmental drawbacks.
**Expert Overall Verdict & Recommendation:** Nitrogen fixation is an indispensable process that underpins life on Earth. Its benefits far outweigh its limitations. Promoting and supporting nitrogen fixation, particularly biological nitrogen fixation, is crucial for sustainable agriculture and environmental stewardship. We highly recommend further research and development in this area.
## Insightful Q&A Section
Here are some frequently asked questions about why plants and animals can’t directly use atmospheric nitrogen, going beyond the basics:
1. **Why can some bacteria fix nitrogen, but not all?**
*Only bacteria with the nitrogenase enzyme are capable of nitrogen fixation. The genes encoding nitrogenase are not universally present in all bacteria due to evolutionary factors and the metabolic cost of maintaining the enzyme.
2. **How does the Haber-Bosch process compare to biological nitrogen fixation in terms of energy efficiency?**
*The Haber-Bosch process is significantly less energy-efficient than biological nitrogen fixation. It requires high temperatures and pressures, consuming a large amount of fossil fuels. Biological nitrogen fixation occurs under ambient conditions and requires less energy per unit of nitrogen fixed.
3. **What are the implications of increased atmospheric nitrogen deposition on ecosystems?**
*Increased nitrogen deposition can lead to eutrophication of aquatic ecosystems, acidification of soils, and loss of biodiversity in terrestrial ecosystems. It can also contribute to the formation of smog and acid rain.
4. **How does climate change affect nitrogen fixation rates?**
*Climate change can affect nitrogen fixation rates in complex ways. Changes in temperature, precipitation patterns, and atmospheric CO₂ concentrations can all influence the activity of nitrogen-fixing organisms. In some cases, nitrogen fixation rates may increase, while in others, they may decrease.
5. **Can genetic engineering be used to create plants that can directly fix nitrogen?**
*While scientists have been working on this for decades, creating plants that can directly fix nitrogen is a major challenge. It would require introducing a complex set of genes encoding nitrogenase and providing the necessary protection from oxygen. Significant progress has been made in transferring nitrogen fixation genes into plants, but fully functional nitrogen-fixing plants are not yet a reality. However, research in 2025 shows promise.
6. **What role do mycorrhizal fungi play in nitrogen uptake by plants?**
*Mycorrhizal fungi form symbiotic relationships with plant roots, enhancing their ability to absorb nutrients, including nitrogen. The fungi extend the reach of the plant’s root system, allowing it to access nitrogen from a larger volume of soil.
7. **How does soil pH affect nitrogen availability to plants?**
*Soil pH affects the solubility of nitrogen compounds and the activity of nitrifying and denitrifying bacteria. Most plants prefer a slightly acidic to neutral soil pH for optimal nitrogen uptake.
8. **What are some strategies for reducing nitrogen losses from agricultural fields?**
*Strategies for reducing nitrogen losses include using slow-release fertilizers, applying fertilizers at the right time and in the right amount, planting cover crops, and implementing conservation tillage practices.
9. **How does the nitrogen cycle interact with other biogeochemical cycles, such as the carbon and phosphorus cycles?**
*The nitrogen cycle is interconnected with other biogeochemical cycles. For example, nitrogen can limit carbon sequestration in terrestrial ecosystems, and phosphorus is essential for nitrogen fixation.
10. **What are the potential risks and benefits of using genetically modified nitrogen-fixing bacteria in agriculture?**
*Genetically modified nitrogen-fixing bacteria could potentially enhance nitrogen fixation rates and reduce the need for synthetic fertilizers. However, there are also potential risks, such as the unintended consequences of releasing genetically modified organisms into the environment.
## Conclusion
In conclusion, the inability of plants and animals to directly utilize atmospheric nitrogen stems from the strong triple bond in the N₂ molecule, requiring specialized biological or industrial processes to break it. The nitrogen cycle, with its intricate steps of fixation, ammonification, nitrification, and denitrification, is nature’s elegant solution to making this essential element accessible to life. While the Haber-Bosch process has revolutionized agriculture, its environmental impacts highlight the importance of sustainable nitrogen management. Understanding these processes is crucial for ensuring food security and protecting our planet. The future of nitrogen management lies in improving nitrogen use efficiency, promoting biological nitrogen fixation, and minimizing nitrogen losses from agricultural fields. Share your experiences with nitrogen management in the comments below. Explore our advanced guide to sustainable agriculture for more insights.
### SEO Title Options:
1. Atmospheric Nitrogen: Why Plants & Animals Can’t Use It
2. N2 Unusable? The Nitrogen Cycle Explained Simply
3. Why Plants Need Fixed Nitrogen: An Expert Guide
4. Can’t Use Air Nitrogen? Plants, Animals & N2 Fixation
5. Nitrogen in Air: Why It’s Useless (Without This!)
### Meta Description:
Why can’t plants and animals use nitrogen directly from the air? Learn the science behind nitrogen fixation, the nitrogen cycle, and the vital role of bacteria! Get expert insights now. #nitrogen #plants #biology