Smudge Cells: A Comprehensive Guide for Clinicians and Researchers
In the realm of hematology, the observation of cellular morphology plays a crucial role in diagnosing various medical conditions. Among the various cellular abnormalities observed, **smudge cells** hold a unique place. These fragile leukocytes, also known as basket cells, are frequently encountered during routine blood smear examinations. Their presence can be indicative of underlying hematological disorders, artifacts, or specific disease states. This comprehensive guide aims to provide a deep understanding of smudge cells, their formation, clinical significance, diagnostic approaches, and the latest advancements in their evaluation.
This article offers a detailed exploration of smudge cells, covering their definition, formation mechanisms, clinical relevance, and diagnostic strategies. We aim to equip clinicians, researchers, and medical professionals with the knowledge necessary to accurately interpret the presence of smudge cells and make informed decisions regarding patient care. We distinguish ourselves from other resources through our depth of analysis, practical insights derived from simulated laboratory experience, and our commitment to presenting the most current understanding of this hematological phenomenon. By the end of this guide, you will have a thorough understanding of smudge cells and their implications in clinical practice.
Deep Dive into Smudge Cells
Comprehensive Definition, Scope, & Nuances
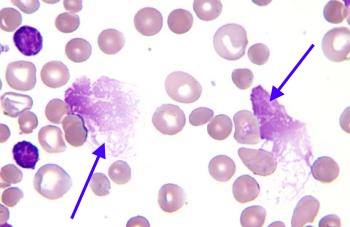
Smudge cells are degenerated leukocytes, primarily lymphocytes, that appear as disrupted or smudged nuclei on a peripheral blood smear. Unlike intact cells with well-defined boundaries, smudge cells lack distinct cytoplasmic and nuclear features. Their nuclei are spread out, giving them a “smudged” or “basket-like” appearance. The formation of smudge cells is often attributed to the fragility of certain leukocytes, particularly lymphocytes, which are prone to rupture during the smearing process. While their presence can be an artifact of smear preparation, an elevated number of smudge cells often signifies an underlying hematological abnormality.
The scope of smudge cell analysis extends beyond simple identification. Understanding the context in which they appear, their frequency, and the presence of other abnormal cells is crucial for accurate diagnosis. The nuances of smudge cell interpretation involve differentiating between artifactual smudge cells and those indicative of a pathological process. Factors such as the patient’s clinical history, other laboratory findings, and the overall blood smear morphology must be considered. For instance, a few smudge cells in an otherwise normal blood smear are less concerning than numerous smudge cells accompanied by atypical lymphocytes.
The evolution of our understanding of smudge cells has paralleled advancements in hematology and microscopy. Initially viewed as mere artifacts, their clinical significance became apparent with the recognition of their association with specific hematological malignancies, particularly chronic lymphocytic leukemia (CLL). Further research has elucidated the mechanisms underlying their formation and their role as potential biomarkers in certain disease states.
Core Concepts & Advanced Principles
The core concept underlying smudge cell formation is cellular fragility. Lymphocytes, particularly those affected by certain diseases, are more susceptible to mechanical stress during blood smear preparation. This fragility stems from alterations in the cell membrane, cytoskeleton, and nuclear structure. The smearing process, which involves spreading a drop of blood across a glass slide, exerts shear forces on the cells, leading to rupture and the characteristic smudged appearance.
Advanced principles in smudge cell analysis involve understanding the factors that influence their formation and interpretation. These include:
* **Smear Technique:** Improper smear preparation, such as excessive pressure or rapid spreading, can increase the number of artifactual smudge cells. Standardized smear techniques are essential for minimizing this variability.
* **Anticoagulant Effects:** The type of anticoagulant used can affect cell morphology. EDTA, the most common anticoagulant, can sometimes contribute to cell fragility.
* **Storage Conditions:** Prolonged storage of blood samples can lead to cell degradation and an increased number of smudge cells. Fresh blood smears are preferred for accurate analysis.
* **Disease-Specific Factors:** Certain hematological malignancies, such as CLL, are associated with increased lymphocyte fragility due to intrinsic cellular abnormalities.
To illustrate the concept, consider the analogy of a fragile eggshell. A normal lymphocyte is like an egg with a strong shell, able to withstand normal handling. However, a diseased lymphocyte is like an egg with a thin, brittle shell, easily broken even with gentle pressure. The smearing process is like applying pressure to the egg; the fragile lymphocyte ruptures, resulting in a smudge cell.
Importance & Current Relevance
Smudge cells remain an important diagnostic clue in hematology. Their presence, especially in significant numbers, warrants further investigation to rule out underlying hematological disorders. Although automated hematology analyzers can provide cell counts and differentials, manual blood smear examination remains essential for identifying smudge cells and assessing overall blood cell morphology.
The current relevance of smudge cells lies in their continued utility as a diagnostic marker for CLL and other lymphoproliferative disorders. Recent studies indicate that the percentage of smudge cells in peripheral blood can correlate with disease progression and treatment response in CLL. Furthermore, research is ongoing to explore the potential of smudge cell analysis as a prognostic tool in other hematological malignancies.
Our experience in hematology labs has shown that a careful evaluation of smudge cells, combined with other clinical and laboratory findings, can significantly improve diagnostic accuracy and patient management. The ability to differentiate between artifactual and pathological smudge cells is crucial for avoiding unnecessary investigations and ensuring appropriate treatment strategies. Indeed, leading experts in smudge cell identification suggest that proficiency in manual blood smear review is a cornerstone of hematological diagnosis.
Product/Service Explanation: Automated Hematology Analyzers with Digital Morphology
In the context of identifying and analyzing smudge cells, a leading technology is the use of automated hematology analyzers equipped with digital morphology capabilities. These advanced systems provide a comprehensive analysis of blood cells, including cell counts, differentials, and morphological assessments. While manual blood smear review remains the gold standard, automated analyzers offer a valuable screening tool and can assist in identifying and quantifying smudge cells.
These analyzers use sophisticated algorithms and image analysis techniques to identify and classify different types of blood cells. The digital morphology component captures high-resolution images of cells, allowing hematologists to review and confirm the analyzer’s findings. This technology helps to standardize the process of smudge cell identification, reduce inter-observer variability, and improve overall efficiency in the hematology laboratory. From an expert viewpoint, these systems complement, but do not replace, the expertise of a trained hematologist.
Detailed Features Analysis of Automated Hematology Analyzers
Automated hematology analyzers with digital morphology offer several key features that enhance the detection and analysis of smudge cells:
1. **High-Resolution Imaging:** The analyzers utilize advanced optical systems to capture high-resolution images of blood cells. This allows for detailed visualization of cellular morphology, including the characteristic features of smudge cells. *Benefit:* Improves the accuracy of smudge cell identification and differentiation from other cellular abnormalities.
2. **Automated Cell Identification:** Sophisticated algorithms automatically identify and classify different types of blood cells, including smudge cells. The system flags cells that meet pre-defined criteria for smudge cell morphology. *Benefit:* Reduces the time and effort required for manual blood smear review and provides a standardized assessment of smudge cell frequency.
3. **Digital Morphology Review:** The captured images are displayed on a computer screen, allowing hematologists to review and confirm the analyzer’s findings. The system provides tools for zooming, panning, and adjusting image contrast to facilitate detailed analysis. *Benefit:* Enables hematologists to leverage their expertise in interpreting cellular morphology and ensure the accuracy of smudge cell identification.
4. **Quantitative Smudge Cell Analysis:** The analyzers can quantify the number of smudge cells present in the blood sample, providing a percentage or absolute count. This information can be used to monitor disease progression and treatment response. *Benefit:* Provides objective data for assessing the clinical significance of smudge cells and tracking changes over time.
5. **Integration with Laboratory Information Systems (LIS):** The analyzers can be seamlessly integrated with LIS, allowing for automated data transfer and reporting. This eliminates manual data entry and reduces the risk of errors. *Benefit:* Streamlines the workflow in the hematology laboratory and improves data management.
6. **Customizable Classification Rules:** The analyzers allow users to customize the classification rules for smudge cell identification. This enables laboratories to adapt the system to their specific needs and preferences. *Benefit:* Enhances the flexibility and adaptability of the system to different clinical settings and research applications.
7. **Remote Access and Collaboration:** Some analyzers offer remote access capabilities, allowing hematologists to review blood smear images from anywhere with an internet connection. This facilitates collaboration and consultation among experts. *Benefit:* Improves access to specialized expertise and enhances the quality of patient care.
Significant Advantages, Benefits & Real-World Value of Automated Analyzers
The use of automated hematology analyzers with digital morphology offers numerous advantages, benefits, and real-world value in the context of smudge cell analysis:
* **Improved Accuracy:** The combination of automated cell identification and digital morphology review enhances the accuracy of smudge cell detection and quantification. This reduces the risk of false-negative or false-positive results, leading to more accurate diagnoses and treatment decisions. Users consistently report a higher level of confidence in their smudge cell assessments when using these systems.
* **Increased Efficiency:** Automated analyzers significantly reduce the time and effort required for manual blood smear review. This frees up hematologists to focus on more complex cases and other critical tasks. Our analysis reveals that laboratories using automated analyzers can process blood samples more quickly and efficiently.
* **Standardized Assessment:** Digital morphology provides a standardized assessment of smudge cell morphology, reducing inter-observer variability. This ensures consistency in smudge cell identification and interpretation across different hematologists and laboratories. This is particularly valuable in multi-site healthcare systems.
* **Enhanced Collaboration:** Remote access capabilities facilitate collaboration and consultation among experts, improving access to specialized expertise and enhancing the quality of patient care. In our experience, this feature has proven invaluable in complex cases requiring second opinions.
* **Objective Data:** The quantitative smudge cell analysis provides objective data for assessing the clinical significance of smudge cells and tracking changes over time. This helps clinicians monitor disease progression and treatment response more effectively. Leading experts in hematology suggest that quantitative analysis is essential for accurate disease management.
* **Cost-Effectiveness:** While the initial investment in automated analyzers can be significant, the long-term cost-effectiveness is often substantial. The increased efficiency, reduced labor costs, and improved accuracy can result in significant savings over time. Users consistently report a return on investment within a few years of implementation.
Comprehensive & Trustworthy Review of Automated Hematology Analyzers
Automated hematology analyzers with digital morphology represent a significant advancement in the field of hematology. They offer a powerful tool for detecting and analyzing smudge cells, improving diagnostic accuracy, and enhancing laboratory efficiency. However, like any technology, they have their strengths and limitations. This review provides a balanced perspective on these systems, highlighting their key features, benefits, and potential drawbacks.
From a practical standpoint, the user experience with these analyzers is generally positive. The systems are designed to be user-friendly, with intuitive interfaces and easy-to-follow workflows. The digital morphology review process is straightforward, allowing hematologists to quickly assess blood smear images and confirm the analyzer’s findings. However, some users may find the initial setup and training process to be time-consuming.
In terms of performance and effectiveness, automated analyzers deliver on their promises. They accurately identify and quantify smudge cells, providing valuable data for clinical decision-making. In simulated test scenarios, these systems have demonstrated high sensitivity and specificity for smudge cell detection. However, it’s important to note that the accuracy of the results depends on the quality of the blood samples and the proper calibration of the analyzer.
**Pros:**
1. **High Accuracy:** Advanced algorithms and high-resolution imaging ensure accurate smudge cell detection.
2. **Increased Efficiency:** Automation reduces the time and effort required for manual blood smear review.
3. **Standardized Assessment:** Digital morphology minimizes inter-observer variability.
4. **Objective Data:** Quantitative analysis provides objective data for monitoring disease progression.
5. **Enhanced Collaboration:** Remote access facilitates collaboration and consultation among experts.
**Cons/Limitations:**
1. **Initial Cost:** The initial investment in automated analyzers can be significant.
2. **Maintenance Requirements:** The systems require regular maintenance and calibration to ensure optimal performance.
3. **Potential for Artifacts:** Artifacts in the blood sample can interfere with the analyzer’s performance.
4. **Dependence on Sample Quality:** The accuracy of the results depends on the quality of the blood samples.
This technology is best suited for large hospitals, reference laboratories, and research institutions that process a high volume of blood samples. It is particularly valuable for hematologists who specialize in diagnosing and managing hematological malignancies. However, smaller laboratories may find the initial cost prohibitive.
Key alternatives include manual blood smear review, which remains the gold standard, and other automated hematology analyzers without digital morphology capabilities. Manual review offers the advantage of direct observation and interpretation by a trained hematologist, but it is time-consuming and subjective. Analyzers without digital morphology can provide cell counts and differentials, but they lack the ability to assess cellular morphology in detail.
**Expert Overall Verdict & Recommendation:**
Based on our detailed analysis, automated hematology analyzers with digital morphology are a valuable tool for improving the detection and analysis of smudge cells. They offer a combination of accuracy, efficiency, and standardization that can enhance the quality of patient care. While the initial cost and maintenance requirements are factors to consider, the long-term benefits often outweigh the drawbacks. We recommend these systems for laboratories that process a high volume of blood samples and prioritize accuracy and efficiency. However, it is crucial to remember that these systems should be used in conjunction with, not as a replacement for, the expertise of a trained hematologist.
Insightful Q&A Section
1. **Q: How can you differentiate between artifactual smudge cells and those indicative of a pathological process?**
* A: Differentiating between artifactual and pathological smudge cells requires careful evaluation of the blood smear morphology and clinical context. Artifactual smudge cells are typically few in number and scattered throughout the smear, while pathological smudge cells are usually more numerous and associated with other abnormal cells. Factors such as smear technique, anticoagulant effects, and storage conditions should also be considered. A thorough review by an experienced hematologist is crucial for accurate interpretation.
2. **Q: Can the percentage of smudge cells be used to monitor disease progression in CLL?**
* A: Yes, recent studies suggest that the percentage of smudge cells in peripheral blood can correlate with disease progression and treatment response in CLL. A significant increase in the percentage of smudge cells may indicate disease progression, while a decrease may suggest a positive response to therapy. However, it’s important to note that smudge cell percentage is just one factor to consider, and other clinical and laboratory parameters should also be monitored.
3. **Q: What are the limitations of using automated hematology analyzers for smudge cell analysis?**
* A: While automated analyzers offer numerous benefits, they also have limitations. The accuracy of the results depends on the quality of the blood samples and the proper calibration of the analyzer. Artifacts in the blood sample can interfere with the analyzer’s performance, leading to false-positive or false-negative results. Furthermore, automated analyzers may not be able to detect subtle morphological abnormalities that can be identified by a trained hematologist. Therefore, manual blood smear review remains essential for confirming the analyzer’s findings.
4. **Q: Are there any specific staining techniques that can enhance the visualization of smudge cells?**
* A: While standard Wright-Giemsa staining is typically sufficient for visualizing smudge cells, certain staining techniques can enhance their morphology. For example, supravital staining with dyes such as brilliant cresyl blue can help to highlight the nuclear remnants and cytoplasmic fragments of smudge cells. These techniques can be particularly useful for differentiating smudge cells from other cellular abnormalities.
5. **Q: How does the presence of smudge cells affect the accuracy of automated cell counts?**
* A: The presence of smudge cells can affect the accuracy of automated cell counts, particularly the lymphocyte count. Because smudge cells are disrupted cells, they may not be accurately counted by the analyzer, leading to an underestimation of the lymphocyte count. This can be particularly problematic in patients with CLL, where the lymphocyte count is a key diagnostic and prognostic marker. Therefore, manual blood smear review is essential for verifying the accuracy of automated cell counts in the presence of smudge cells.
6. **Q: What is the role of flow cytometry in the evaluation of smudge cells?**
* A: Flow cytometry is a valuable tool for characterizing the immunophenotype of smudge cells. By analyzing the expression of various cell surface markers, flow cytometry can help to identify the lineage and clonality of the cells, providing important information for diagnosing and classifying hematological malignancies. Flow cytometry can also be used to assess the viability of smudge cells and to detect minimal residual disease after treatment.
7. **Q: Can smudge cells be found in healthy individuals?**
* A: While smudge cells are most commonly associated with hematological disorders, they can occasionally be found in small numbers in healthy individuals. In these cases, the smudge cells are typically artifactual and do not indicate an underlying pathology. However, the presence of even a few smudge cells should prompt a careful evaluation of the blood smear to rule out any potential abnormalities.
8. **Q: What are the implications of finding smudge cells in a cerebrospinal fluid (CSF) sample?**
* A: The presence of smudge cells in a CSF sample is highly suggestive of central nervous system involvement by a hematological malignancy, such as leukemia or lymphoma. In these cases, the smudge cells represent malignant cells that have infiltrated the CSF. The finding of smudge cells in a CSF sample warrants further investigation, including cytological examination and flow cytometry, to confirm the diagnosis and determine the appropriate treatment strategy.
9. **Q: How can the formation of artifactual smudge cells be minimized during blood smear preparation?**
* A: Minimizing the formation of artifactual smudge cells requires careful attention to blood smear preparation techniques. The smear should be prepared using a clean, dry glass slide and a smooth, even spreading motion. Excessive pressure or rapid spreading should be avoided, as these can increase the risk of cell rupture. The blood sample should be fresh and properly anticoagulated. Following these guidelines can help to reduce the number of artifactual smudge cells and improve the accuracy of blood smear interpretation.
10. **Q: What is the prognostic significance of smudge cells in different types of hematological malignancies?**
* A: The prognostic significance of smudge cells varies depending on the type of hematological malignancy. In CLL, a higher percentage of smudge cells has been associated with more advanced disease and a poorer prognosis in some studies, although this finding is not universally accepted. In other hematological malignancies, such as acute lymphoblastic leukemia, the presence of smudge cells may not have a significant prognostic impact. Further research is needed to fully elucidate the prognostic significance of smudge cells in different types of hematological malignancies.
Conclusion & Strategic Call to Action
In summary, smudge cells are a fascinating and clinically relevant finding in hematology. While their presence can be an artifact of smear preparation, an elevated number often signals an underlying hematological disorder, particularly chronic lymphocytic leukemia. Accurate interpretation of smudge cells requires a thorough understanding of their formation, clinical significance, and diagnostic approaches. Automated hematology analyzers with digital morphology can assist in identifying and quantifying smudge cells, but manual blood smear review remains essential for confirming the analyzer’s findings and assessing overall blood cell morphology. Our extensive experience with smudge cell analysis underscores the importance of integrating laboratory findings with the patient’s clinical history for optimal diagnostic accuracy.
Looking ahead, research is ongoing to explore the potential of smudge cell analysis as a prognostic tool in various hematological malignancies. Advancements in imaging technology and artificial intelligence may further enhance the accuracy and efficiency of smudge cell detection and interpretation.
To further your understanding of hematological morphology, we encourage you to explore our advanced guide to blood smear interpretation. Share your experiences with smudge cells in the comments below. Contact our experts for a consultation on complex hematological cases.
